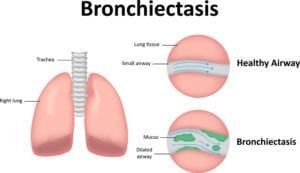
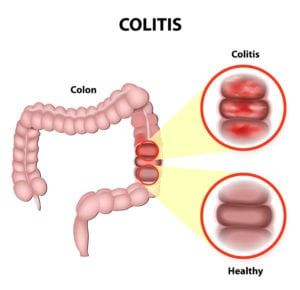
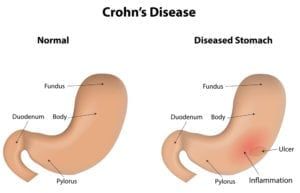
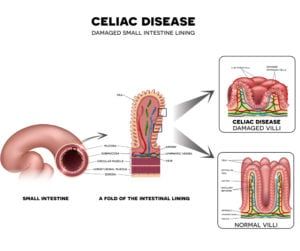
What is compounding?
Compounding is known as the process of combining, mixing, and altering two or more ingredients to create a medication customized to the patient’s needs. Compounded drugs can help treat patients who cannot be treated with an FDA approved medication, such as allergies to certain dyes, and those who cannot swallow capsules. Some examples of compounding include:
- Flavoring a medication for a child or pet
- Customizing the strength of the dose
- Reformulating the drug to exclude unwanted ingredients
These compounds can be made into flavored liquids, topical creams, transdermal gels, and other dosage forms for patients’ needs.
Why is pharmaceutical compounding needed?
Manufacturing, and traditional compounding does not always accommodate the patient’s needs. If a health care provider prescribes a drug that’s only commercially available in an oral format, for instance, patients can ask their prescriber about whether or not compounded medications can be available for their needs.
How is pharmaceutical compounding different from traditional compounding?
Traditional compounding follows the exact specifications of the medication, and manufacturing these drugs is typically mass-produced by the FDA, then sold to pharmacies, health care practitioners, and other medical professionals authorized under law to resell these drugs. Pharmaceutical compounding is performed under the state’s pharmacy board and operates under a different set of legal rules.
What laws regulate compounding pharmacies?
Pharmaceutical compounds follow the FDA’s regulations outlined under Section 503A and the Compounding Quality Act and are subjected to oversight under federal and state authorities, but do not follow the CGMP regulations regarding manufacturing, processing, and drug packaging products.. Pharmaceutical compounds are also overseen by the Drug Enforcement Administration, which regulates controlled substances in use for compounded medications, such as narcotics, amphetamines, and drugs used for sleeping and anxiety disorders. Other organizations, such as the United States Pharmacopeial Convention, issue standards to define purity, quality, and identification of drugs used in compounded medications.
Who can administer compounded medications?
Patients can receive compounded medications from a community pharmacy, specialty pharmacy, doctor, or other health professionals in medical offices and clinics. Patients can ask the administer whether the drug is compounded or manufactured, and can ask whether the administer is accredited before receiving the compounded medication. Outsourcing facilities can also create compounding drugs and are overseen by the Drug Quality and Security Act and are annually inspected by the FDA according to a risk-based schedule.
What are the risks associated with compounded drugs?
Because each compounded medication isn’t overseen by the CGMP guidelines, the FDA does not verify the compounded drugs’ safety and effectiveness. If poor compounding practices exist, it can lead to serious drug quality problems, leading to an increased risk of patient injury or death. For higher quality standards, other FDA regulations are applied according to the setting where compounding occurs.
If you have any concerns regarding our compounding medications, our safety policies, and our practice, please contact our office about our compounding services.